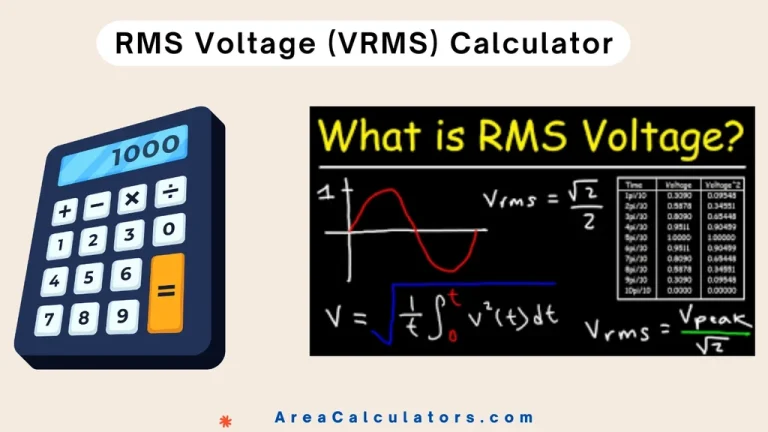
Binding Energy Per Nucleon Calculator
Use our basic and advanced calculators for quick calculations !
The Binding Energy Per Nucleon Calculator helps you determine the amount of energy required to disassemble a nucleus. In order to understand nuclear stability, this tool is too cruicial. Elements like Fe-56 have the highest binding energy per nucleon, making them the most stable. This calculator simplifies calculating nuclear binding energies for isotopes like Uranium-235 and Uranium-238.

Formula:
The formula is:
| Variable | Meaning |
|---|---|
| BE | Binding Energy Per Nucleon (energy required to bind a nucleus together) |
| Δm | Mass Defect (difference between the mass of the nucleus and its components) |
| c | Speed of Light (constant, approximately ) |
| A | Mass Number (the total number of protons and neutrons in the nucleus) |
How to Calculate ?
To calculate the Binding Energy Per Nucleon (BE):
- Multiply the mass defect (Δm) by the square of the speed of light (c²).
- Divide the result by the mass number (A), which is the total number of protons and neutrons in the nucleus. This gives the binding energy per nucleon, which indicates how tightly the nucleons (protons and neutrons) are held together in a nucleus.
Solved Calculations :
Example 1:
Given:
- Mass Defect (Δm) = 0.01 kg
- Speed of Light (c) = m/s
- Mass Number (A) = 56
| Calculation | Instructions |
|---|---|
| Step 1: BE = | Start with the formula. |
| Step 2: BE = | Replace Δm with 0.01 kg, c with m/s, and A with 56. |
| Step 3: BE = | Square the speed of light. |
| Step 4: BE = | Multiply the mass defect by |
| Step 5: BE = | Divide by the mass number (56) to get the binding energy per nucleon. |
Answer:
The binding energy per nucleon is .
Example 2:
Given:
- Mass Defect (Δm) = 0.02 kg
- Speed of Light (c) = m/s
- Mass Number (A) = 100
| Calculation | Instructions |
|---|---|
| Step 1: BE = | Start with the formula. |
| Step 2: BE = | Replace Δm with 0.02 kg, c with m/s, and A with 100. |
| Step 3: BE = | Square the speed of light. |
| Step 4: BE = | Multiply the mass defect by . |
| Step 5: BE = | Divide by the mass number (100) to get the binding energy per nucleon. |
Answer:
The binding energy per nucleon is .
What is Binding Energy Per Nucleon Calculator?
Binding Energy Per Nucleon Calculator lets you know the energy required to separate a nucleus into its individual nucleons. It is a crucial concept in nuclear physics and chemistry that helps us tounderstand nuclear stability. It is calculated using the mass defect (the difference between the mass of a nucleus and the sum of its constituent protons and neutrons) and Einstein’s equation, E=mc².
The binding energy per nucleon is typically expressed in MeV and can be computed by dividing the total binding energy of the nucleus by the number of nucleons it contains.
Uranium-235 and Uranium-238 have different binding energies due to their unique nucleon configurations. The highest binding energy per nucleon is found in Iron-56 (Fe-56), which is the most stable nucleus, thereby, it requires the most energy to break apart. This is reflected in the binding energy per nucleon chart, which shows the variation in nuclear stability across elements. The binding energy per nucleon graph peaks around Fe-56, explaining why heavy elements like uranium are less stable and prone to fission.
To calculate binding energy per nucleon, you need the mass defect and apply the formula in MeV. The curve of binding energy per nucleon indicates the most stable nuclei, making it a vital tool in nuclear energy calculations.
![Centipoise To Centistokes Calculator [ Viscosity, Poise Stokes ] 2 Calculator for calculating surface area, volume, and dimensions of various shapes and objects.](https://areacalculators.com/wp-content/uploads/2025/07/centipoise-to-centistokes-calculator-768x432.webp)