RF Value Calculator
To calculate the Rf value in chromatography, measure the distance the solute travels from the origin and divide it by the distance the solvent front travels. This simple division provides the retention factor, or Rf, value.
To calculate the Rf value in chromatography, measure the distance the solute travels from the origin and divide it by the distance the solvent front travels. This simple division provides the retention factor, or Rf, value.
In chromatography, the Rf value (retention factor) is integral for analyzing and comparing substances. It serves to identify compounds based on their movement across a stationary phase relative to a solvent. Different compounds have unique Rf values due to factors like polarity and solubility.
For instance, pigments or amino acids will have varying Rf values that indicate their purity and identity in the mixture. Scientists and researchers use these values to examine chemical behavior and properties in fields like biochemistry, pharmaceuticals, and environmental studies.
| Symbol | Description |
|---|---|
| DSU | Distance traveled by the solute |
| DSV | Distance traveled by the solvent front |
Example 1:
Suppose the solute travels 3 cm, and the solvent front reaches 6 cm.
| Step | Calculation |
|---|---|
| Solute Distance (DSU) | 3 cm |
| Solvent Distance (DSV) | 6 cm |
| Rf Calculation | |
| Result | 0.5 |
Answer: The Rf value is 0.5.
Example 2:
If the solute covers 5 cm and the solvent front 10 cm:
| Step | Calculation |
|---|---|
| Solute Distance (DSU) | 5 cm |
| Solvent Distance (DSV) | 10 cm |
| Rf Calculation | |
| Result | 0.5 |
Answer: The Rf value is 0.5.
The RF Value Calculator is a retention factor measuring tool. It is specifically used in chromatography for finding out the retention factor or RF value. This factor measures how far a compound travels relative to the solvent front on a chromatography plate.
This value is instrumental in analyzing and identifying various substances. As a matter of fact, different compounds have unique RF values based on factors like polarity, solvent type, and interaction with the stationary phase.
To calculate the RF value, you can use the formula RF = Distance traveled by the compound / Distance traveled by the solvent front.
For example, if a compound moves 2 cm on the TLC plate and the solvent front moves 5 cm, the RF value is 0.4. This tool enables precise and efficient calculation of RF values, making it easier to analyze chromatography results.
In a word, the RF Value Calculator simplifies chromatography analysis, providing accurate RF values to assist in identifying compounds and assessing purity in mixtures.
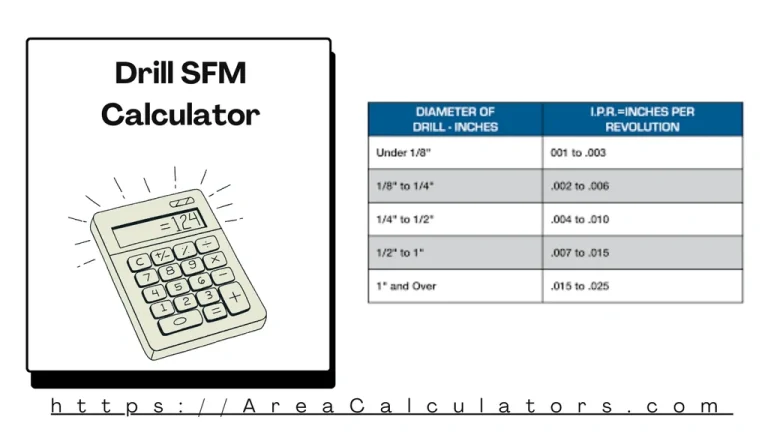
To determine the surface feet per minute (SFM) for drilling, multiply the cutting speed by the diameter of the drill and convert the result into feet per minute using a standard formula. The Drill SFM Calculator is a vital tool for machinists and engineers to optimize drilling operations by calculating the surface feet per minute…
Divide the standard time or target time to complete a task by the actual time taken, then multiply by 100 for the efficiency percentage. Time efficiency measures how effectively a task is completed within a given timeframe. It is particularly useful in industries like manufacturing, logistics, and project management where timely delivery is crucial. By…
To estimate battery run time, multiply the battery’s ampere-hour rating by 10, then divide by the device’s wattage. The Battery Run Time Calculator is a handy tool for gauging how long a battery will last when powering a specific device. Knowing battery runtime helps you plan power usage, whether for gadgets, vehicles, or backup systems….

13 / 100 SEO Score Welcome to our 1095 Rule Calculator. Enter the required data, in both basic and advanced calculator for accurate calculation. Further read our solved examples and formula to get better understanding Formula: The formula is: C=(D1095)×100C = \left(\frac{D}{1095}\right) \times 100 Variables: Variable Meaning C Compliance Percentage D Number of Days Covered…
To calculate the cost per gallon, divide the total cost of the liquid (fuel, water, etc.) by the total volume in gallons. The Cost Per Gallon Calculator is an efficient tool for calculating the cost of liquid commodities like fuel, water, or gas. It is particularly beneficial for managing budgets, estimating expenses, or comparing prices during…
7 / 100 SEO Score To calculate the coefficient of coincidence (COC), sum the product of each individual frequency multiplied by one less than that frequency, and then divide by the total number of pairs in the population. The coefficient of coincidence is a genetic term used to measure the occurrence of double crossover events…